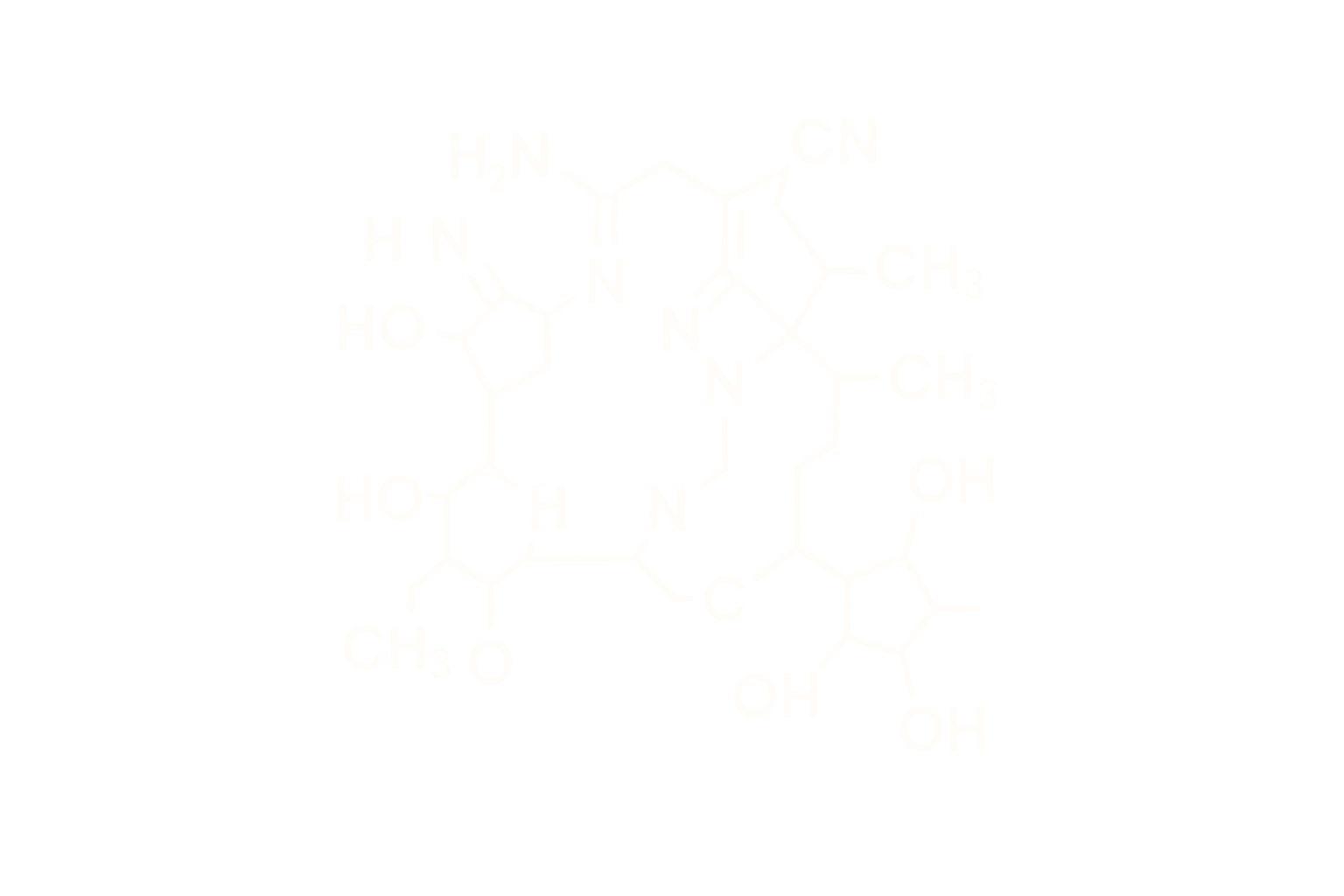
Tesamorelin – Stabilized Synthetic GHRH Analog
Chemical Identity
Chemical Name: Tesamorelin Acetate
Synonym: ThGRF(1–44)NH2
Molecular Formula: C₂₂₁H₃₆₆N₇₂O₆₇S
Molecular Weight: ~5135.91 Da
CAS Number: 218949-48-5
Sequence: A full-length synthetic growth hormone-releasing factor (1–44) analog with a trans-3-hexenoic acid (Th) group at the N-terminus
Structure Type: Synthetic stabilized peptide analog of human GHRH (1–44)
Pharmacological Classification
Tesamorelin is a **synthetic stabilized growth hormone-releasing hormone (GHRH) analog** designed to increase endogenous pulsatile secretion of growth hormone (GH). It binds to the **GHRH receptor (GHRHR)** and is the only GHRH analog approved by the FDA for the treatment of HIV-associated lipodystrophy due to its lipolytic and IGF-1 elevating properties.
Mechanism of Action
- GHRHR Agonism: Binds and activates the GHRH receptor in the anterior pituitary to stimulate GH release.
- Stabilization via N-Terminal Modification: Addition of a trans-3-hexenoic acid moiety increases resistance to proteolytic cleavage (e.g., DPP-IV) and improves pharmacokinetics.
- Feedback Preserved: Enhances endogenous GH rhythm without suppressing hypothalamic feedback mechanisms.
β-Arrestin Recruitment
Tesamorelin, like native GHRH(1–44), exhibits **moderate β-arrestin recruitment** at the GHRHR. It activates canonical cAMP-PKA signaling while also triggering internalization and receptor recycling pathways. Compared to short GHRH fragments, Tesamorelin demonstrates stronger arrestin-associated signaling.
GHRH Receptor Signaling Profile
| Ligand | GHRHR Binding | cAMP Activation | β-Arrestin Recruitment |
|---|---|---|---|
| GHRH (1–44) | Yes | High | Moderate |
| Tesamorelin | Yes | High | Moderate |
| Mod-GRF (1–29) | Yes | High | Low |
Pharmacokinetics (Non-Dosing)
- Half-Life: ~26–38 minutes (longer than GHRH)
- Bioavailability: Effective via subcutaneous injection with extended activity vs. GHRH
- Clearance: Primarily proteolytic degradation and renal excretion
Biological Effects
Elevates serum GH and IGF-1, enhances lipolysis, improves metabolic profiles, and reduces visceral adipose tissue in HIV-associated lipodystrophy. Studies also suggest potential utility in cognitive aging and cardiovascular health due to systemic IGF-1 upregulation.
Stability and Storage
- Form: Lyophilized acetate salt
- Solubility: Sterile water, saline, or buffered diluents (pH 4.5–6.0)
- Storage: –20°C; protect from light and moisture
- Reconstitution: Prepare in sterile environment and refrigerate if stored
References
- Falutz J, et al. J Clin Endocrinol Metab. 2007;92(11):4265–4274.
- Stanley TL, et al. AIDS. 2014;28(9):1339–1346.
- Grunfeld C, et al. Ann Intern Med. 2010;153(9):633–642.
- Thorner MO, et al. J Clin Endocrinol Metab. 1992;75(2):528–533.