Retatrutide – Triple GIP/GLP-1/Glucagon Receptor Agonist
Chemical Identity
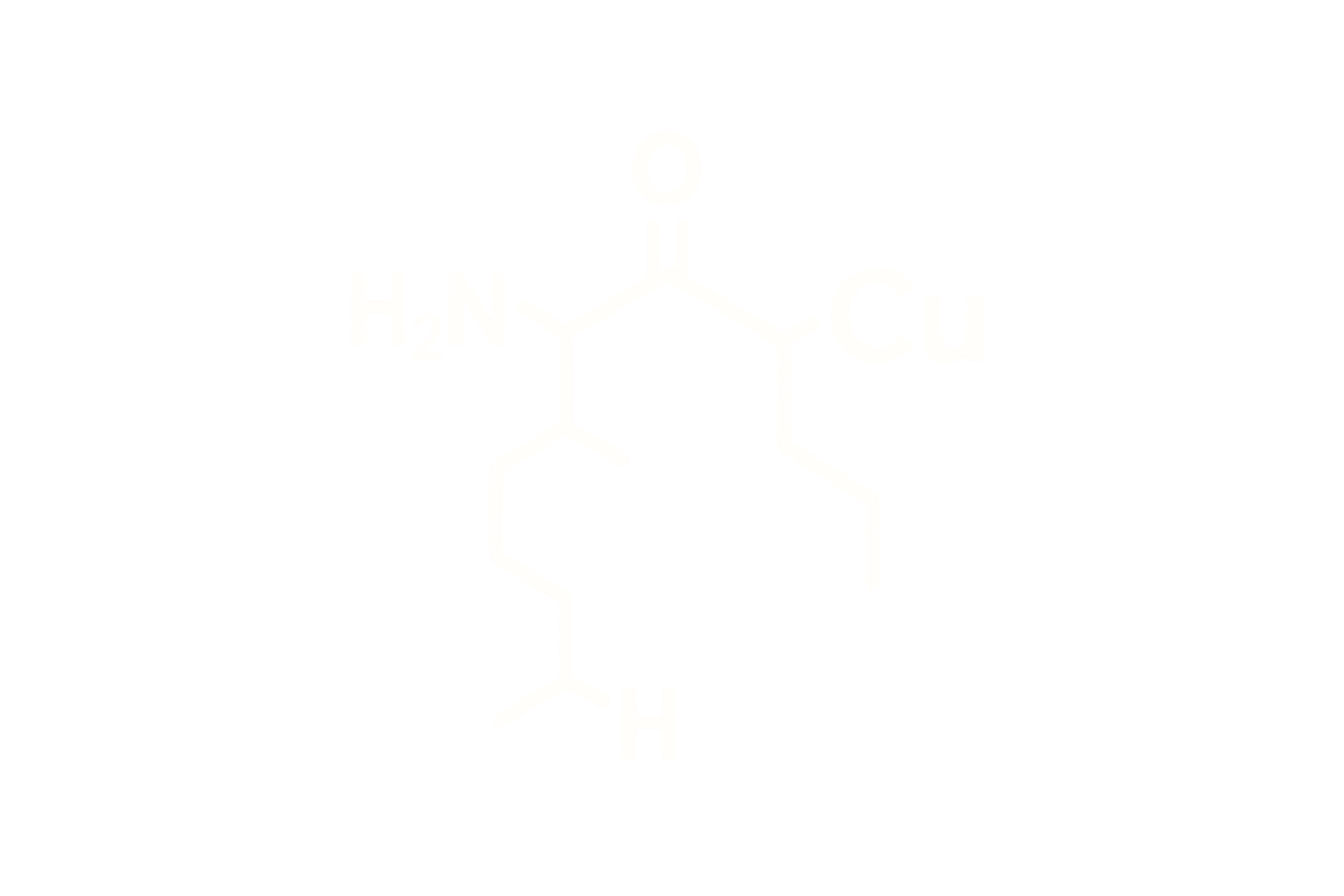
Chemical Name: Retatrutide Acetate
Molecular Formula: C₂₂₁H₃₄₃N₄₇O₆₇
Molecular Weight: ~4761.6 Da
CAS Number: 2762296-94-4
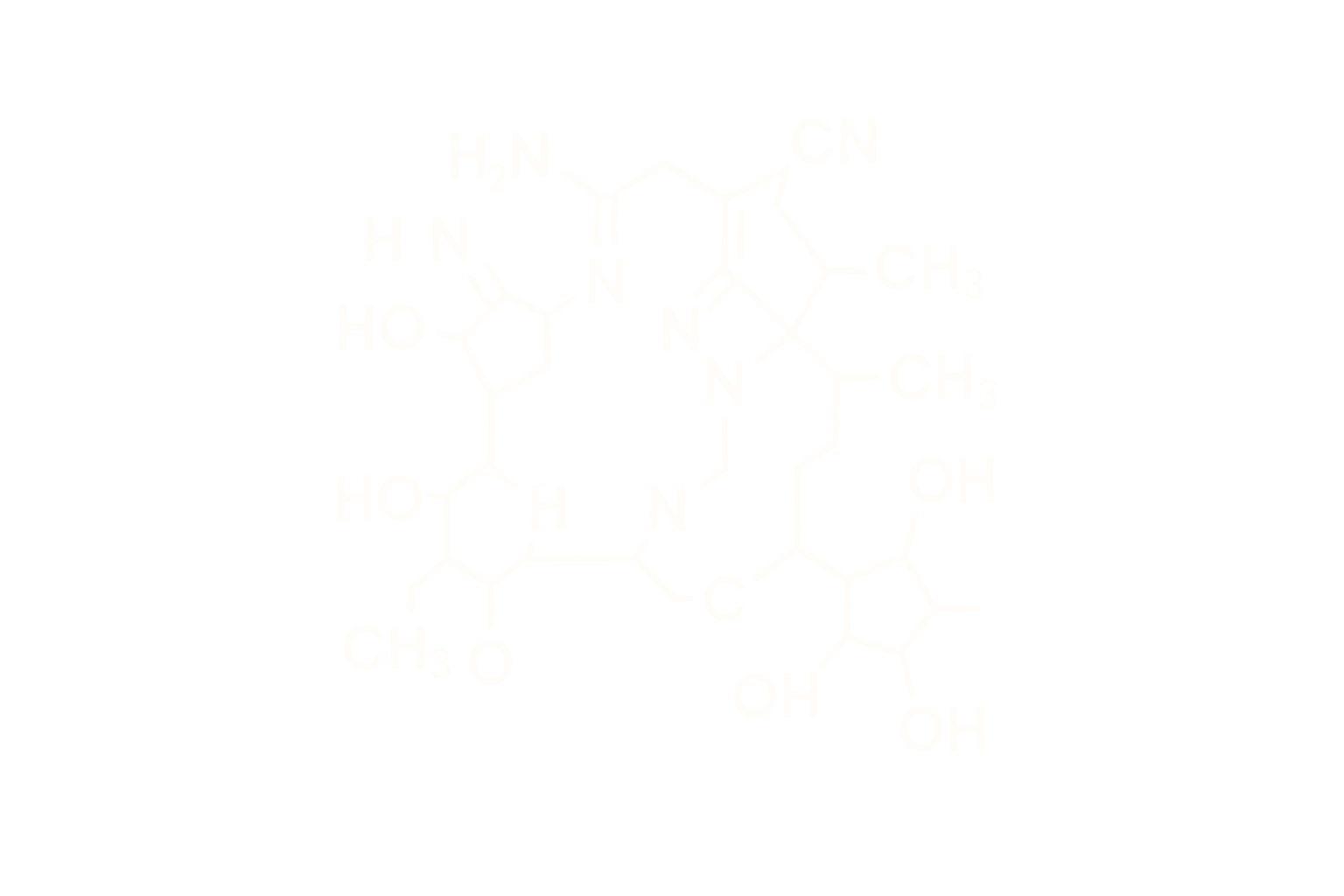
Sequence (Linear): Proprietary sequence engineered for balanced co-activation of GIPR, GLP-1R, and GCGR, with a C20 fatty diacid side chain at a lysine residue
Modifications: Multisite substitution and lipidation for DPP-IV resistance and extended circulation
Structure Type: Synthetic linear peptide, 39 amino acids, lyophilized acetate salt
Pharmacological Classification
Retatrutide is a **balanced triple agonist** of the GIP, GLP-1, and glucagon receptors. It represents a next-generation incretin-based therapeutic platform designed to maximize metabolic flexibility through multi-receptor synergy.
Mechanism of Action
- GIPR Activation: Enhances insulin secretion, adipocyte glucose uptake, and energy storage in a glucose-dependent manner.
- GLP-1R Activation: Suppresses appetite, increases satiety, slows gastric emptying, and improves β-cell responsiveness.
- GCGR Activation: Stimulates hepatic fatty acid oxidation and thermogenesis; increases energy expenditure.
- Signaling: All three receptors signal via Gs-mediated cAMP accumulation; downstream effects are receptor- and tissue-specific.
β-Arrestin Recruitment
Retatrutide exhibits tailored β-arrestin profiles across each of its three target receptors:
- GLP-1R: Acts as a biased agonist favoring cAMP production over β-arrestin recruitment, reducing receptor internalization and extending signaling duration.
- GIPR: Moderate β-arrestin recruitment consistent with endogenous GIP activity.
- GCGR: Exhibits balanced recruitment to support controlled receptor trafficking and avoid overstimulation.
Comparative β-Arrestin Profile
| Receptor | Native Ligand | β-Arrestin Recruitment | Retatrutide Activity |
|---|---|---|---|
| GLP-1R | GLP-1 | High | Low (biased toward cAMP) |
| GIPR | GIP | Moderate | Similar |
| GCGR | Glucagon | Moderate | Balanced |
References: Kelly AS et al., NEJM 2023;389(7):621–632. Willard FS et al., Cell Reports 2020;33(6):108293. Nestorowicz T et al., bioRxiv 2023.
Molecular Engineering
Engineered through strategic residue substitutions and site-specific lipidation to optimize receptor binding, prolong half-life, and maintain GIPR/GLP-1R/GCGR signaling balance. DPP-IV-resistant design ensures metabolic stability.
Pharmacokinetics (Non-Dosing)
- Elimination: Hepatic and renal degradation of peptide fragments
- Plasma Protein Binding: >99% via albumin-binding fatty acid moiety
- Half-Life: ~5–6 days (estimated from Phase 1 data)
Biological Effects
Combines glycemic control (GLP-1/GIP) with weight loss and energy expenditure enhancement (GCGR). In animal and human studies, leads to reductions in body fat, fasting insulin, triglycerides, and hepatic steatosis.
Preclinical Evidence
In rodent DIO models and non-human primates, retatrutide reduced total body weight by up to 24%, exceeding effects of GLP-1 mono- or dual agonists. Demonstrated favorable cardiovascular and hepatic metabolic markers.
Stability and Storage
- Form: Lyophilized peptide acetate salt
- Solubility: Water, acetate buffer (pH 4–6), DMSO for analytical prep
- Storage: –20°C; protected from light and moisture
- Reconstitution pH: 4.0–5.5 preferred
Comparative Mechanistic Summary
| Parameter | GLP-1 | GIP | Glucagon | Retatrutide |
|---|---|---|---|---|
| Receptor Targets | GLP-1R | GIPR | GCGR | All three |
| β-arrestin Recruitment | High | Moderate | Moderate | Low–Moderate |
| Half-life | Minutes | Minutes | Minutes | ~5–6 days |
| Albumin Binding | No | No | No | Yes |
References
- Kelly AS, et al. NEJM. 2023;389(7):621–632.
- Nestorowicz T, et al. bioRxiv. 2023. doi:10.1101/2023.07.05.548892
- Willard FS, et al. Cell Reports. 2020;33(6):108293.
- Finan B, et al. Cell Metab. 2019;29(3):740–750.e10.
- Coskun T, et al. Diabetes. 2018;67(Supplement_1):64-LB.