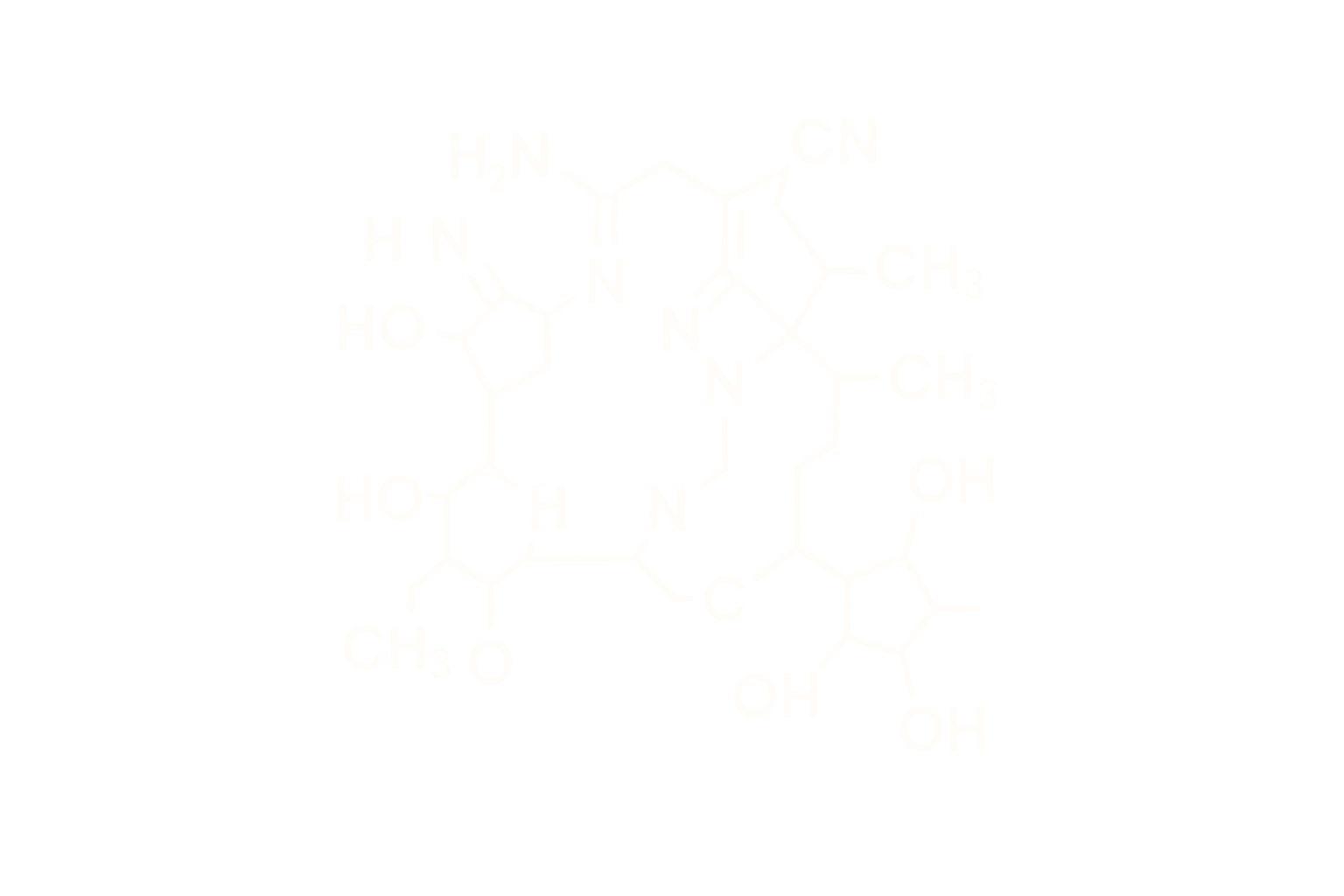
Mod-GRF (1–29) – Tetrasubstituted GHRH Analog (CJC-1295 without DAC)
Chemical Identity
Chemical Name: Modified GRF (1–29) Acetate
Also Known As: CJC-1295 without DAC, tetrasubstituted GRF(1–29)
Molecular Formula: C₁₅₂H₂₅₂N₄₄O₄₂S
Molecular Weight: ~3367.9 Da
CAS Number: 863288-34-0
Sequence (Modified): Tyr-D-Ala-Asp-Ala-Ile-Phe-Thr-Asn-Ser-Tyr-Arg-Lys-Val-Leu-Ala-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Ile-Met-Ser-Arg-NH2
Structure Type: Synthetic 29-amino acid peptide with substitutions at positions 2 (D-Ala), 8 (Ala), 15 (Leu), and 27 (Met) for DPP-IV resistance
Pharmacological Classification
Mod-GRF (1–29) is a **short-acting analog of growth hormone-releasing hormone (GHRH)** engineered for increased stability and bioavailability. It activates the **GHRH receptor (GHRHR)** in pituitary somatotrophs, triggering physiological GH pulses without long-term receptor downregulation.
Mechanism of Action
- GHRHR Binding: Activates Gs-coupled signaling leading to increased cAMP and GH release.
- Tetrasubstitution: Enhances resistance to enzymatic degradation by DPP-IV, extending the peptide’s in vivo activity.
- Pulsatile Modulation: Amplifies endogenous growth hormone rhythm without altering hypothalamic-pituitary feedback.
β-Arrestin Recruitment
Mod-GRF (1–29) demonstrates **low β-arrestin recruitment** at the GHRH receptor. This supports a favorable bias toward cAMP production over receptor internalization, reducing desensitization risk compared to full-length GHRH (1–44).
GHRH Receptor Signaling Profile
| Ligand | Receptor Binding | cAMP Activation | β-Arrestin Recruitment |
|---|---|---|---|
| GHRH (1–44) | Yes | High | Moderate |
| Mod-GRF (1–29) | Yes | High | Low |
Pharmacokinetics (Non-Dosing)
- Half-Life: ~30 minutes in vivo (short-acting)
- Administration: Rapid subcutaneous absorption; short systemic exposure
- Clearance: Proteolytic degradation via peptidases; not albumin-bound
Biological Effects
Stimulates pulsatile growth hormone release and indirectly raises IGF-1. Supports muscle preservation, recovery, and tissue remodeling. Unlike DAC-conjugated analogs, Mod-GRF allows for more physiologic timing and pairing with short-acting secretagogues (e.g., Ipamorelin).
Stability and Storage
- Form: Lyophilized acetate salt
- Solubility: Water, acetate buffer, dilute HCl (pH 4.0–6.0)
- Storage: –20°C; avoid light and moisture
- Reconstitution: Sterile water or saline recommended
References
- Teichman SL, et al. J Clin Endocrinol Metab. 2006;91(11):4734–4743.
- Rahim A, et al. Clin Endocrinol. 1996;44(1):69–75.
- Ross RJM, et al. J Endocrinol. 2001;171(2):R1–R5.
- CJC-1295 Patent WO2003043581A1 – DAC and tetrasubstitution claims