Peptide Manufacturing and Salt Form Comparison
Peptide therapeutics are typically produced using solid-phase peptide synthesis (SPPS), a method involving the sequential coupling of protected amino acids onto a resin support. Once the desired peptide sequence is complete, cleavage from the resin is performed using a strong acid—commonly trifluoroacetic acid (TFA)—which also removes side-chain protecting groups. The resulting crude peptide contains TFA as a counterion, forming a TFA salt.
While effective in synthesis, residual TFA poses significant drawbacks, including cytotoxicity, volatility, poor solubility, and chemical instability. To enhance purity and performance, peptides are often converted into more suitable salt forms via ion exchange, resulting in acetate, hydrochloride (HCl), sodium, or formate salts depending on the formulation goal.
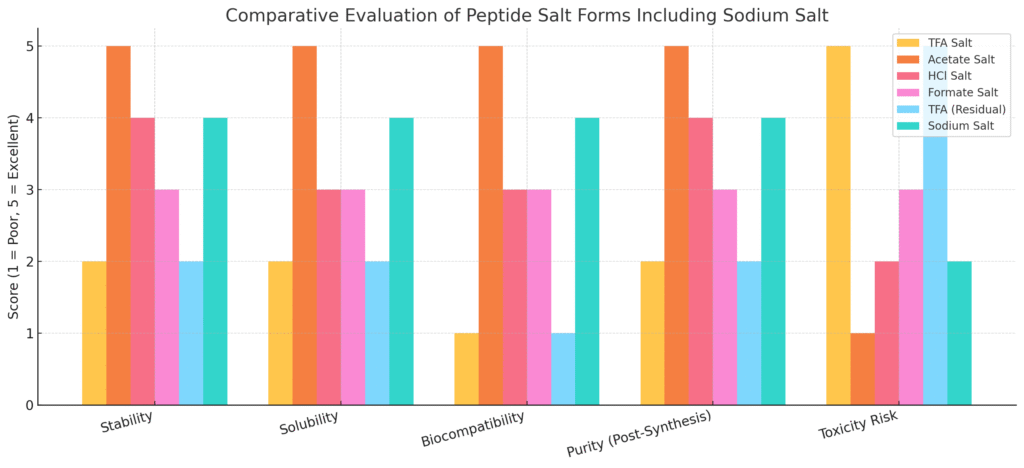
Salt Form Overview and Comparison
| Salt Form | Stability | Solubility | Biocompatibility | Purity Enhancement | Toxicity Risk |
|---|---|---|---|---|---|
| Acetate | Excellent | Excellent | High | High | Low |
| Sodium | Good | Good | High | High | Low |
| Hydrochloride (HCl) | Moderate | Moderate | Moderate | Moderate | Moderate |
| Formate | Moderate | Moderate | Moderate | Moderate | Low |
| TFA (Residual) | Poor | Poor | Low | Low | High |
Why Acetate is Preferred
Among all salt forms, acetate salts offer the best balance of chemical stability, aqueous solubility, physiological safety, and reproducible purity. Acetic acid is a naturally occurring short-chain fatty acid that poses no risk of immunogenicity, and when reconstituted with bacteriostatic water or saline, the resulting solution mirrors the tonicity and pH of physiological fluids.
The ion-exchange step used to replace TFA with acetate eliminates cytotoxic residues and supports downstream lyophilization, storage, and reconstitution. These advantages make acetate the gold standard for research-grade peptide formulations.
Sodium Salts and Pharmaceutical Buffering
Sodium salts are another high-performance option, widely used in injectable medications and IV buffers. Sodium acetate in particular is used in sodium acetate injection, USP to treat metabolic acidosis and is an essential component of dialysis solutions. Sodium salts form stable, clear solutions when reconstituted and often resemble saline in osmolarity and pH.
While sodium salts may be slightly less stable than acetate in freeze-dried form, they are well-tolerated and easy to prepare, especially for aqueous formulations.
Conclusion
Choosing the correct salt form is critical in peptide research and formulation. Acetate and sodium salts are superior for stability, compatibility, and purity. In contrast, TFA salts, while synthetically convenient, are not recommended for use in sensitive biological systems due to toxicity concerns. Proper salt selection enhances solubility, reduces side effects, and ensures consistent experimental outcomes.