Peptide Manufacturing, Formulation, and Lyophilization
The production of high-purity peptides for research and pharmaceutical use is a multistep process involving chemical synthesis, purification, counter-ion exchange, stabilization, sterile filtration, and lyophilization. Each stage plays a critical role in preserving peptide integrity and ensuring reproducibility.
1. Solid-Phase Peptide Synthesis (SPPS)
Peptides are synthesized via SPPS using Fmoc- or t-Boc-based chemistries. Amino acids are sequentially attached to a solid resin with precise control over sequence and modifications.
2. Cleavage and Deprotection
Once synthesis is complete, the peptide is cleaved from the resin and protective groups are removed using trifluoroacetic acid (TFA) or other acid cocktails.
3. Crude Purification
Crude peptides are purified using preparative HPLC to eliminate side-products, deletion sequences, and unreacted fragments.
4. TFA Removal and Counter-ion Exchange
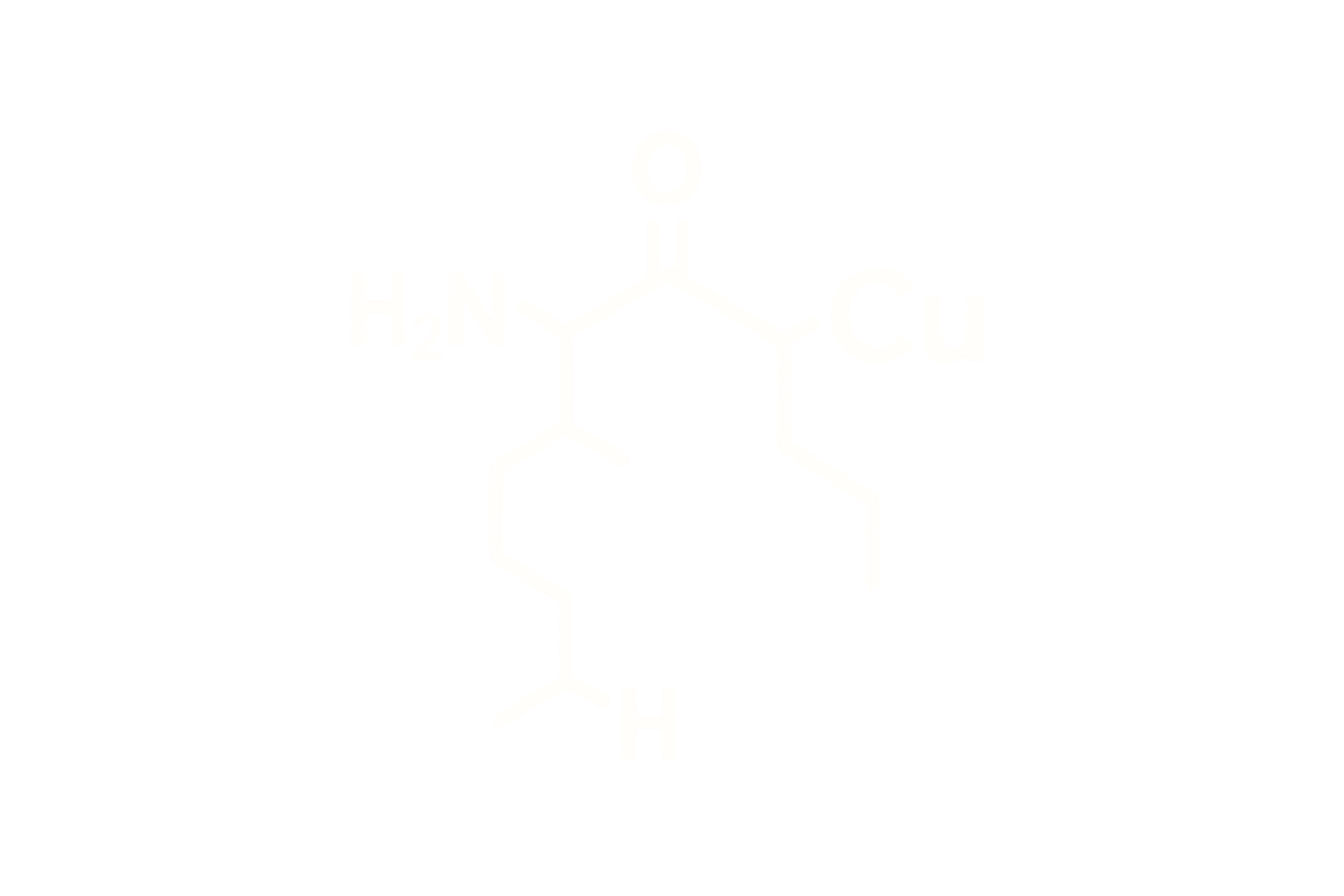
TFA is replaced with acetate through a buffer exchange process using acetic acid or sodium acetate. Acetate salts improve stability and reduce cytotoxicity compared to TFA or hydrochloride forms.
5. Final Purification and Characterization
High-resolution HPLC and mass spectrometry confirm identity and purity, typically targeting ≥98% for research-grade peptides.
6. Addition of Bulking Agents and Excipients
Bulking agents such as mannitol or trehalose are added to ensure sufficient fill volume and cake structure during freeze-drying. Sodium acetate or other buffers help stabilize pH.
7. Sterile Filtration
The peptide formulation is passed through a 0.22 µm sterile filter to eliminate microbial contaminants prior to vialing.
8. Vial Filling and Lyophilization
The sterile peptide solution is filled into vials in an ISO-class cleanroom and rapidly frozen. Under vacuum, water is removed via sublimation, preserving structural integrity in a dry, cake-like matrix.
9. Quality Control and Labeling
Final QC includes purity confirmation, sterility testing, and residual solvent analysis. Vials are labeled and distributed as research-use-only materials.
Why Acetate Salts and Bulking Agents Matter
Acetate salts are favored in peptide formulation due to their physiological compatibility and improved solubility. Sodium acetate is widely accepted in injectable formulations. Mannitol is the preferred bulking agent due to its inert properties and ability to maintain cake structure and reconstitution ease post-lyophilization.
When executed with precision, this process results in stable, high-purity peptides suitable for rigorous scientific investigation and therapeutic development.
📊 Peptide Processing Workflow – Step-by-Step Overview
| Step | Process Description |
|---|---|
| 🧪 Peptide Synthesis | Automated SPPS using Fmoc/t-Boc chemistry on solid-phase resins. |
| ⚗️ Cleavage & Deprotection | TFA is used to cleave and remove protecting groups from the peptide. |
| 🌀 Crude Purification | Initial RP-HPLC purification to isolate target peptide. |
| 💨 TFA Removal | TFA is removed and replaced with acetate via buffer exchange. |
| 🔄 Counter-ion Exchange | Peptide converted to acetate salt using acetic acid or sodium acetate. |
| 🧼 Final Purification | Further purification (≥98%) and characterization via MS/HPLC. |
| 🍚 Bulking Agents | Mannitol or trehalose added to aid freeze-drying and structure. |
| 🧴 Buffering & Stabilization | Sodium acetate added to stabilize pH and improve shelf-life. |
| 🧼 Sterile Filtration | 0.22 µm filtration ensures sterility before vialing. |
| 💉 Vial Distribution | Filtered peptide is filled into sterile vials in ISO cleanrooms. |
| ❄️ Lyophilization | Vacuum freeze-drying yields a dry, stable peptide cake. |
| ✅ QC & Labeling | Final testing for purity, sterility, solvents; labeled for research use only. |
Peptide Manufacturing – Simplified Overview
The process of making high-quality peptides for research involves many careful steps to ensure purity, stability, and consistency. It all starts with building the peptide chain one amino acid at a time using a method called solid-phase peptide synthesis (SPPS). This allows scientists to create the exact sequence they need.
Once the chain is complete, it’s removed from the solid support and cleaned of any extra chemical pieces using a strong acid like TFA. This results in a crude mixture that needs to be purified. Using special chromatography techniques, the target peptide is separated from unwanted by-products.
After that, the TFA is removed and replaced with a safer and more stable form—usually an acetate salt. The peptide is purified again to reach very high levels of purity, often above 98%.
Next, bulking agents like mannitol or trehalose are added. These help form a solid structure during the drying process and make the product easier to handle and store. Buffers may also be added to keep the pH stable.
The final liquid is then passed through a sterile filter to remove any potential contaminants and filled into clean, sterile vials. Finally, the vials go through freeze-drying (lyophilization), where the liquid is frozen and gently dried under vacuum. This leaves behind a dry powder that’s stable, easy to store, and ready to be reconstituted when needed.
Every batch goes through strict quality checks before it’s labeled and released for research use.